 DON’T WAIT —
DON’T WAIT —
Studies show importance of acting early
A critical point occurs when dogs suffering from congestive heart failure (CHF) begin to show clinical signs. As seen here, following the onset of clinical signs of CHF, progression to death is rapid.
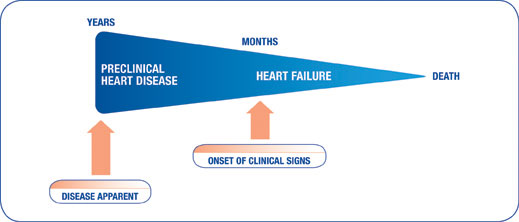
As a result, at the appearance of the first clinical signs of CHF, therapeutic intervention is indicated.1 Appropriate and rapid treatment of clinical CHF, with standard treatment of a diuretic, ACE inhibitor and VETMEDIN, can improve the dog’s quality and duration of life.1-4
Studies show benefits of early VETMEDIN use in dogs with CHF1-4
The VetSCOPE* study results support an early therapeutic role for VETMEDIN.1 In the VetSCOPE trial, clinical signs in dogs treated with VETMEDIN improved significantly by Day 7 and continued to improve throughout the trial. At Day 56, dogs treated with VETMEDIN showed significant improvement in exercise tolerance, demeanor, and respiratory effort. Dogs were able to enjoy the benefits of treatment quickly and experienced continued, steady improvement in clinical signs.1
Similarly, results of the QUEST* study provide evidence that early treatment helps lengthen life.2 Dogs treated with VETMEDIN lived virtually twice as long as the ACE inhibitor–treated dogs (267 days vs. 140 days) in the QUEST trial, suggesting that earlier treatment with VETMEDIN helps extend life.
The ACVIM recommends treating dogs with VETMEDIN at the first appearance of clinical signs of CHF. View latest ACVIM Cardiology Panel Consensus Statement5
Important safety information
The safety of VETMEDIN has not been established in dogs with asymptomatic heart disease or in heart failure caused by etiologies other than atrioventricular valvular insufficiency or dilated cardiomyopathy. The most common side effects reported in field studies were poor appetite, lethargy, diarrhea, dyspnea, azotemia, weakness, and ataxia. If side effects should occur, pet owners should contact their veterinarian. Please refer to the Full Prescribing Information here.
*Clinical studies were completed using VETMEDIN capsules. In the US, only the chewable tablets are licensed. Both the capsules and chewable tablets contain the same pharmaceutical ingredient, pimobendan, and are considered equivalent for clinical use. Bioequivalence, however, has not been shown.
References:
1. Lombard CW, Jöns O, Bussadori CM; for the VetSCOPE Study. Clinical efficacy of pimobendan versus benazepril for the treatment of acquired atrioventricular valvular disease in dogs. J Am Anim Hosp Assoc. 2006;42(4):249–261. 2. Häggström J, Boswood A, O’Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22(5):1124–1135. 3. O’Grady MR, Minors SL, O’Sullivan ML, Horne R. Effect of pimobendan on case fatality rate in Doberman pinschers with congestive heart failure caused by dilated cardiomyopathy. J Vet Intern Med. 2008;22(4):897– 904. 4. Luis Fuentes V, Corcoran B, French A, Schober KE, Kleemann R, Justus C. A double-blind, randomized, placebo-controlled study of pimobendan in dogs with dilated cardiomyopathy. J Vet Intern Med. 2002;16(3):255– 261. 5. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23(6):1142–1150.

 VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.
VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.