 Congestive heart failure
Congestive heart failure
Inevitably, atrioventricular valvular insufficiency (AVVI) and dilated cardiomyopathy (DCM) will likely result in congestive heart failure (CHF). Heart failure occurs when the heart, weakened by disease, is unable to pump enough blood to sufficiently meet the body’s needs.
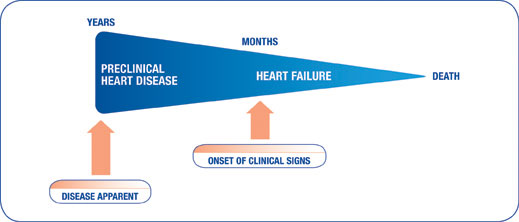
The progression of CHF
The progression of heart failure varies on a case-by-case basis and in particular by disease. With AVVI, there is a long preclinical period in which damage to the heart valves occurs over years, whereas with DCM the preclinical course can vary from months to years.
Ultimately, though, following the onset of CHF, there is a rapid decline towards death that can occur in a matter of months if no treatment is initiated.
Treatment with VETMEDIN at the onset of CHF will help dogs feel better and live longer.1,2
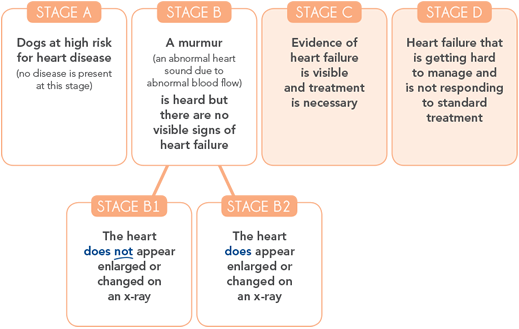
ACVIM recommendations
Dogs with CHF can only progress in one direction—from heart disease to heart failure. Recent treatment guidelines from the American College of Veterinary Internal Medicine (ACVIM) now recommend use of VETMEDIN at the onset of clinical signs of CHF.3 Click here to see the 2009 ACVIM Consensus Statement.
Important safety information
Use only in dogs with clinical evidence of heart failure. The safety of VETMEDIN has not been established in dogs with asymptomatic heart disease or in heart failure caused by etiologies other than atrioventricular valvular insufficiency or dilated cardiomyopathy.
References:
1. Lombard CW, Jöns O, Bussadori CM; for the VetSCOPE Study. Clinical efficacy of pimobendan versus benazepril for the treatment of acquired atrioventricular valvular disease in dogs. J Am Anim Hosp Assoc. 2006;42(4):249–261. 2. Häggström J, Boswood A, O’Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22(5):1124–1135. 3. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23(6):1142–1150.

 VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.
VETMEDIN is a registered trademark of Boehringer Ingelheim Vetmedica GmbH,
licensed to Boehringer Ingelheim Vetmedica, Inc.